We are coauthors on a new paper out in PNAS that uses a new genome for a shrew-like mammal to study patterns of venom evolution in vertebrates.
Congratulations to lab member Daren Card, first author Nick Casewell, and all co-authors!
Casewell N.R., D. Petras, D.C. Card, V. Suranse, A.M. Mychajliw, D. Richards, I. Koludarov, L.-O. Albulescu, J. Slagboom, B.-F. Hempel, N.M. Ngum, R.J. Kennerley, J.L. Brocca, G. Whiteley, R.A. Harrison, F.M. Bolton, J. Debono, F.J. Vonk, J. Alföldi, J. Johnson, E. Karlsson, K. Lindblad-Toh, I. Mellor, R.D. Süssmuth, B.G. Fry BG, S. Kuruppu, W.C. Hodgson, J. Kool, T.A. Castoe, I. Barnes, K. Sunagar, E.A.B. Undheim and S.T. Turvey. 2019. Solenodon genome reveals convergent evolution of venom in eulipotyphlan mammals. Proceedings of the National Academy of Sciences USA. Early Online edition: https://doi.org/10.1073/pnas.1906117116. PDF
Abstract
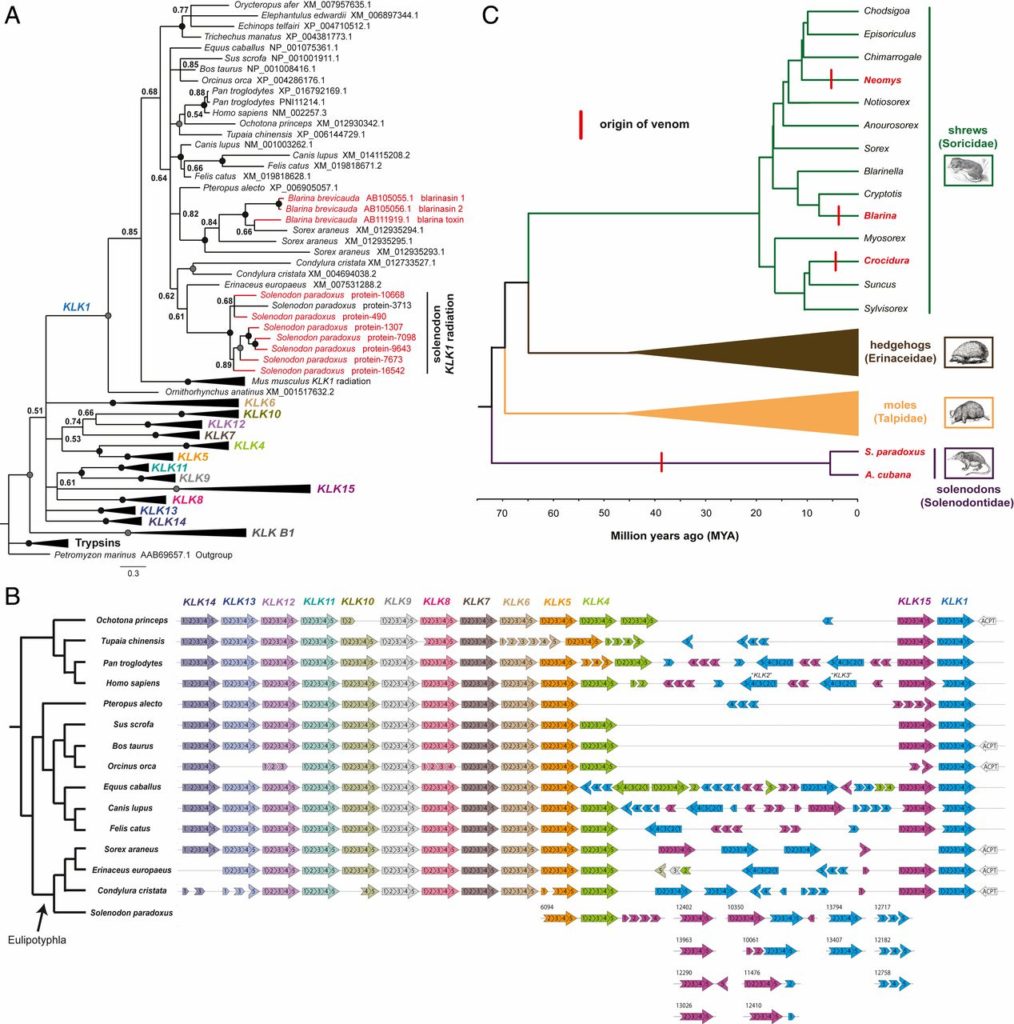
Venom systems are key adaptations that have evolved throughout the tree of life and typically facilitate predation or defense. Despite venoms being model systems for studying a variety of evolutionary and physiological processes, many taxonomic groups remain understudied, including venomous mammals. Within the order Eulipotyphla, multiple shrew species and solenodons have oral venom systems. Despite morphological variation of their delivery systems, it remains unclear whether venom represents the ancestral state in this group or is the result of multiple independent origins. We investigated the origin and evolution of venom in eulipotyphlans by characterizing the venom system of the endangered Hispaniolan solenodon (Solenodon paradoxus). We constructed a genome to underpin proteomic identifications of solenodon venom toxins, before undertaking evolutionary analyses of those constituents, and functional assessments of the secreted venom. Our findings show that solenodon venom consists of multiple paralogous kallikrein 1 (KLK1) serine proteases, which cause hypotensive effects in vivo, and seem likely to have evolved to facilitate vertebrate prey capture. Comparative analyses provide convincing evidence that the oral venom systems of solenodons and shrews have evolved convergently, with the 4 independent origins of venom in eulipotyphlans outnumbering all other venom origins in mammals. We find that KLK1s have been independently coopted into the venom of shrews and solenodons following their divergence during the late Cretaceous, suggesting that evolutionary constraints may be acting on these genes. Consequently, our findings represent a striking example of convergent molecular evolution and demonstrate that distinct structural backgrounds can yield equivalent functions.
Excerpts from the press release
As outlined in a paper published today in PNAS, the team focused their attention on an unusual endangered species known as the Hispaniolan solenodon (Solenodon paradoxus) – a member of the eulipotyphlan order of mammals, an ancient group of insectivores also including hedgehogs, moles and shrews. Obtaining venom from wild solenodons and unravelling the genetic blueprint of this species enabled the identification of the proteins that make up their venom, revealing that it consists of multiple kallikrein-1 serine proteases. Analysis of these toxins showed they are probably used by solenodons to cause drops in blood pressure in the vertebrate prey on which they sometimes feed.
To their surprise, the team found that the kallikrein-1 serine protease toxins found in solenodon venom had evolved in parallel with those detected in the venom of distantly related venomous shrews. The same building blocks of venom have therefore evolved convergently within solenodons and shrews, despite their divergence from one another over 70 million years ago – when dinosaurs still walked the earth.
Professor Nick Casewell from LSTM’s Centre for Snakebite Research & Interventions is lead author on the paper. He said: “These particular proteins are present in the salivary glands of many mammals. Through our research we are able to demonstrate that they have been independently co-opted for a toxic role in the oral venom systems of both solenodons and shrews. These findings represent a fascinating example of how evolution can funnel novel adaptations down repeatable pathways”.
The Hispaniolan solenodon is only found on the Caribbean island of Hispaniola (made up of the Dominican Republic and Haiti), and is considered one of the most Evolutionarily Distinct and Globally Endangered (EDGE) mammals by ZSL’s EDGE of Existence programme. It is one of the few venomous mammals, producing toxic saliva that it injects into its prey through unique grooves in its lower incisor teeth (which gives the solenodon its name). Solenodons are some of the last few surviving Caribbean land mammals and are threatened today by habitat loss and predation from introduced dogs and cats.